Did you know?
If you can answer yes to any of the following questions, you should consult a spine specialist:- Has your low back pain extended down your leg?
- Does your leg pain increase if you lift your knee to your chest or bend over?
- Have you had severe back pain following a recent fall?
- Have you had significant back pain lasting for more than 3 weeks?
- Have you had back pain that becomes worse when you rest, or wakes you up at night?
- Do you have persistent bladder or bowel problems?
Popular subjects
DISC | research | DDD |surgeons | spine | HERNIATED | ARTIFICIAL nucleus | TRIALS | BACK PAIN | video | FUSION | laminectomy | conservative | TREATMENTS
Artificial Disc Replacement
FDA APPROVED, PRODISC®-Lumbar & PRODISC®-Cervical Total Disc Replacement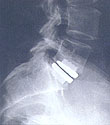 The Spine Institute offers total disc replacement with the ProDisc® implant. The aim of total disc replacement is to recreate normal dynamic function. It can be considered for patients with low back and neck pain as an alternative to spinal fusion.
The Spine Institute offers total disc replacement with the ProDisc® implant. The aim of total disc replacement is to recreate normal dynamic function. It can be considered for patients with low back and neck pain as an alternative to spinal fusion.
What is the PRODISC® Total Disc Replacement?
The PRODISC® Total Disc Replacement is an artificial intervertebral (check FDA approval) disc made from metal and plastic that is used to treat pain associated with degenerative disc disease (DDD).
DDD is defined as discogenic back pain (pain resulting from a degenerated intervertebral disc) with degeneration of the disc confirmed by patient history and radiographic studies.
The PRODISC® Total Disc Replacement is implanted to replace a diseased or damaged intervertebral disc during a surgical procedure called spinal arthroplasty.
How does the PRODISC® Total Disc Replacement work?
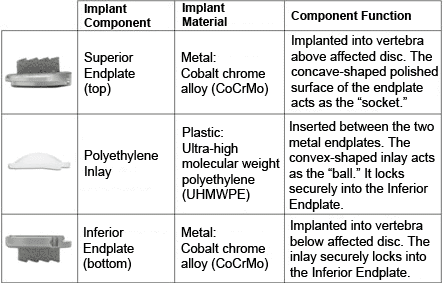
The PRODISC® Total Disc Replacement consists of three parts:
1. Two metal (cobalt-chrome alloy) endplates that are anchored to the top and bottom surfaces of the spinal bones (vertebrae)
2. A plastic (ultra-high molecular weight polyethylene, or UHMWPE) inlay that fits between the two endplates
3. The plastic inlay and endplates help restore the natural distance between the two vertebrae (disc height). The top (superior) endplate can slide over the domed part of the inlay, which can allow movement at the level where it is implanted.

When is the PRODISC® Total Disc Replacement used?
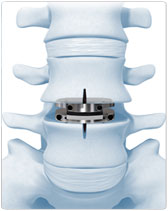
The PRODISC®-L Total Disc Replacement is indicated for spinal arthroplasty in patients who:
- are skeletally mature
- have degenerative disc disease (DDD) at one level in the lumbar spine (from L3-S1) or at one level in the cervical spine (from C3-C7)
- have no more than Grade 1 spondylolisthesis at the involved level
- have had no relief from pain after at least six months of non-surgical treatment
The device may restore disc height, may reduce pain, and may allow movement at the level where it is implanted. The basic principle of the PRODISC® is to restore at least the natural range of mobility and stability in terms of: flexion and extension, lateral bending, and axial rotation and compression.
Up to 12 years of clinical history demonstrates that the ProDisc® can remain mechanically stable, and provide significant pain relief while maintaining mobility.
Read the FDA Approval order #P050010
for the PRODISC®-Lumbar Total Disc Replacement
Read the FDA Approval order #P070001
for the PRODISC®-Cervical Total Disc Replacement
Latest news
Visit our media library for access to all of our news videos.
The Spine Institute is often in the news pioneering new treatments to help the reported 34 million Americans 18 years and older who suffer lower back pain, and another 9 million who suffer neck pain. Watch the news coverage here.
Additional Resources
- NOTE: This list provides links to external on-line resources. Each link will open a new browser window.
- North American Spine Society a non-profit corporation
- Cervical Spine Research Society
- Scoliosis Research Society
- The International Spine Intervention Society
- American Academy of Pain Medicine
- American Pain Society
- A-O North America
- American Academy of Physical Medicine and Rehabilitation
- American Academy of Orthopaedic Surgeons
- American Association of Neurological Surgeons
- Back.com
- Neck Reference
- iScoliosis


- Notice of Disclaimer for external resource links.
The Spine Institute does not support nor endorse any of the resources listed above. They are provided for informational purposes only, and is not a comprehensive list.
